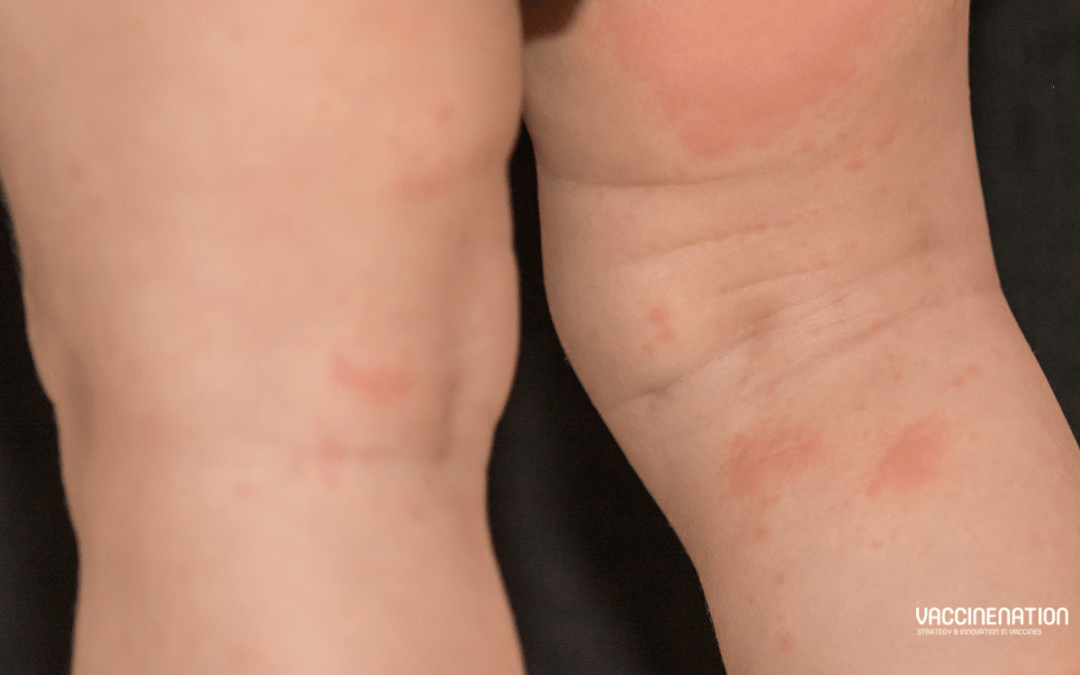
Importance of measles double dose for C-section babies
In May 2024 the University of Cambridge shared that research published in Nature Microbiology (not open access) in partnership with Fudan University, China, finds that a single dose of the measles jab is “up to 2.6 times more likely” to be “completely ineffective” in...

Novavax and Sanofi enter COVID and flu licensing agreement
In May 2024, Novavax and Sanofi announced that they have entered an agreement to co-commercialise Novavax’s COVID-19 vaccine and develop novel flu-COVID-19 combination vaccines. The agreement is intended to provide “broader access” to the protein-based non-mRNA...
Takeda and AC Immune sign agreement on Alzheimer’s vaccine
Takeda announced in May 2024 that it has entered an exclusive, worldwide option and license agreement with AC Immune for the latter’s active
MOST POPULAR THIS MONTH



Science will win, says Valneva’s Thomas Lingelbach
Next from our series of conversations with experts at The World Vaccine Congress in Washington this April is our interview with Valneva's Thomas Lingelbach, who joined us again to share some exciting updates on the team's progress in various pursuits. Thomas is...



Experts say “perspective” needed on AstraZeneca withdrawal
After the announcement that the AstraZeneca COVID vaccine has been withdrawn from the market, public health experts weighed in on media attention that conflated the latest move with a public admission by the company that the vaccine can, in rare cases, cause side...



Representative recruitment is key for Dr Michael Caldwell
Our next interview from The World Vaccine Congress in Washington this April is with Dr Michael C Caldwell of Meharry Medical College, who joined us to chair the Clinical Trials sessions. Dr Caldwell an internal medicine physician with a background in preventative...



Saudi Arabia reports 3 human cases of MERS-CoV in April
In May 2024 WHO issued a Disease Outbreak News (DON) update stating that it has been notified of three human cases, including one death, of Middle East respiratory syndrome coronavirus (MERS-CoV). These cases were reported between 10th and 17th April 2024 by the...



UKHSA data show pertussis cases still increasing
In May 2024, the UKHSA shared that recent data reveal cases of whooping cough “continue to increase”, with the highest burden born by infants. 1,319 cases were confirmed in March, an increase on January’s 556 cases and February’s 918 cases. This takes the total number...



Partnering for pigs: Pirbright and TVG tackle ASF
In May 2024 the Pirbright Institute announced a partnership with researchers at The Vaccine Group on a project that “sets out to control African swine fever” (ASF). ASF is widely prevalent and presents a “significant threat to domestic swine and wild boar...



Broadening challenge studies: hVIVO’s Dr Andrew Catchpole
During our time in Washington for The World Vaccine Congress in April we were glad to meet some of our experts for exclusive conversations about their work. One of these experts is Dr Andrew Catchpole, Chief Scientific Officer at hVIVO; we were delighted that he was...



Vertical transmission milestones for the Americas
In May 2024 PAHO celebrated the latest countries in the Americas that have received WHO certification of the elimination of mother-to-child transmission of HIV and syphilis (EMTCT). This certification is awarded to countries that have: Brought the mother-to-child...


Vaccine “fatigue”: drop in NHS worker flu vaccine uptake
In May 2024 the Local Democracy Reporting Service (LDRS) told the BBC that a decrease in flu vaccine uptake is being attributed to vaccine “fatigue”.


Measuring progress: data for action with EI’s Latifat Okara
Our next conversation from The World Vaccine Congress in Washington is with Economist Impact’s Latifat Okara, who joined us to Chair the Market Access


Infrastructure for innovation with AAHI’s Candice Decaire
Our next interview from our time at the Congress in Washington is a conversation with AAHI’s Candice Decaire, who joined us at the event for a session



Quartet Nanocages against different coronaviruses in study
In May 2024, researchers at the University of Cambridge stated that they have developed a new vaccine technology to provide protection against


Conversations across the law continuum: Brian Dean Abramson
The next in our series of interviews with experts from The World Vaccine Congress in Washington features Brian Dean Abramson of the National Vaccine



WHO and IDIA renew agreement for health impact
In May 2024, WHO and the International Development Innovation Alliance (IDIA) announced the renewal of their strategic Collaborative Agreement
THERAPEUTIC



Takeda and AC Immune sign agreement on Alzheimer’s vaccine
Takeda announced in May 2024 that it has entered an exclusive, worldwide option and license agreement with AC Immune for the latter’s active


“Fierce” response with mRNA vaccine against glioblastoma
Researchers at the University of Florida shared in May 2024 that in a first in human trial of four adult patients, an mRNA cancer vaccine “quickly


UK begins trial of personalised mRNA against melanoma
In April 2024 we learnt of a trial of a personalised mRNA vaccine against the most dangerous form of skin cancer, melanoma. Recruitment for the University


Gritstone shares “positive” data in ongoing GRANITE trial
In April 2024 Gritstone bio announced “positive” preliminary data from an ongoing Phase II portion of the Phase II/III study to evaluate GRANITE.


Geneos announces positive data in GT-30 clinical trial
In April 2024 Geneos Therapeutics announced the publication of “positive” safety, immunogenicity, and efficacy data from a full cohort of patients
TECHNOLOGY


Novavax and Sanofi enter COVID and flu licensing agreement
In May 2024, Novavax and Sanofi announced that they have entered an agreement to co-commercialise Novavax’s COVID-19 vaccine and develop



Broadening challenge studies: hVIVO’s Dr Andrew Catchpole
During our time in Washington for The World Vaccine Congress in April we were glad to meet some of our experts for exclusive conversations about their work.



Quartet Nanocages against different coronaviruses in study
In May 2024, researchers at the University of Cambridge stated that they have developed a new vaccine technology to provide protection against



WHO and IDIA renew agreement for health impact
In May 2024, WHO and the International Development Innovation Alliance (IDIA) announced the renewal of their strategic Collaborative Agreement


Science, accelerated: Q² Solutions’ Dr Luc Gagnon
At this year’s World Vaccine Congress in Washington we were privileged to meet some of our vaccine experts, to hear their insights and learn more
INFECTION



Saudi Arabia reports 3 human cases of MERS-CoV in April
In May 2024 WHO issued a Disease Outbreak News (DON) update stating that it has been notified of three human cases, including one death,



UKHSA data show pertussis cases still increasing
In May 2024, the UKHSA shared that recent data reveal cases of whooping cough “continue to increase”, with the highest burden born by infants



Vertical transmission milestones for the Americas
In May 2024 PAHO celebrated the latest countries in the Americas that have received WHO certification of the elimination of mother-to-child transmission


United in the fight: DRC battles growing mpox outbreak
In April 2024 Ministers of Health and partners in Africa met in Kinshasa, Democratic Republic of Congo, for a high-level emergency regional meeting


Avian influenza in milk and a joint risk assessment
A joint risk assessment from FAO/WHO/WOAH in April 2024 considers the public health risk and risk of virus spread among animals for the A(H5N1)
GLOBAL HEALTH
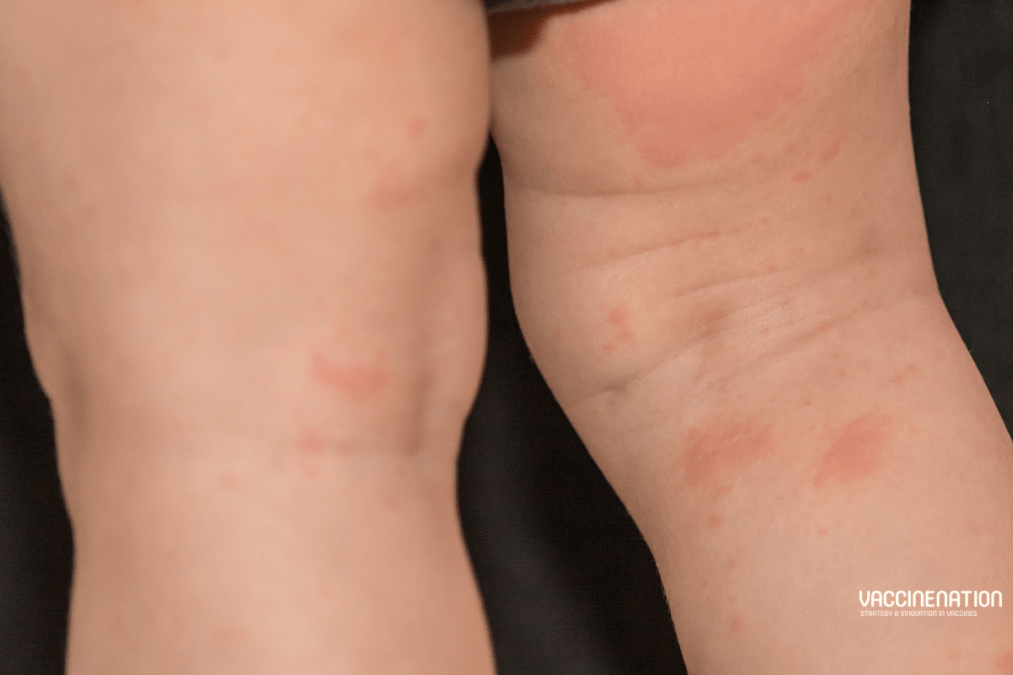

Importance of measles double dose for C-section babies
In May 2024 the University of Cambridge shared that research published in Nature Microbiology (not open access) in partnership with Fudan University,



Science will win, says Valneva’s Thomas Lingelbach
Next from our series of conversations with experts at The World Vaccine Congress in Washington this April is our interview with Valneva’s Thomas Lingelbach,



Experts say “perspective” needed on AstraZeneca withdrawal
After the announcement that the AstraZeneca COVID vaccine has been withdrawn from the market, public health experts weighed in on media



Representative recruitment is key for Dr Michael Caldwell
Our next interview from The World Vaccine Congress in Washington this April is with Dr Michael Caldwell of Meharry Medical College, who joined



Partnering for pigs: Pirbright and TVG tackle ASF
In May 2024 the Pirbright Institute announced a partnership with researchers at The Vaccine Group on a project that “sets out to control African swine fever


